Three-Dimensional Structure of a Streptomyces Sviceus Gnat Acetyltransferase with Similarity to the C-Terminal Domain of the Human Gh84 O-Glcnacase
He, Y., Roth, C., Turkenburg, J.P., Davies, G.J.(2014) Acta Crystallogr D Biol Crystallogr 70: 186
- PubMed: 24419391
- DOI: https://doi.org/10.1107/S1399004713029155
- Primary Citation of Related Structures:
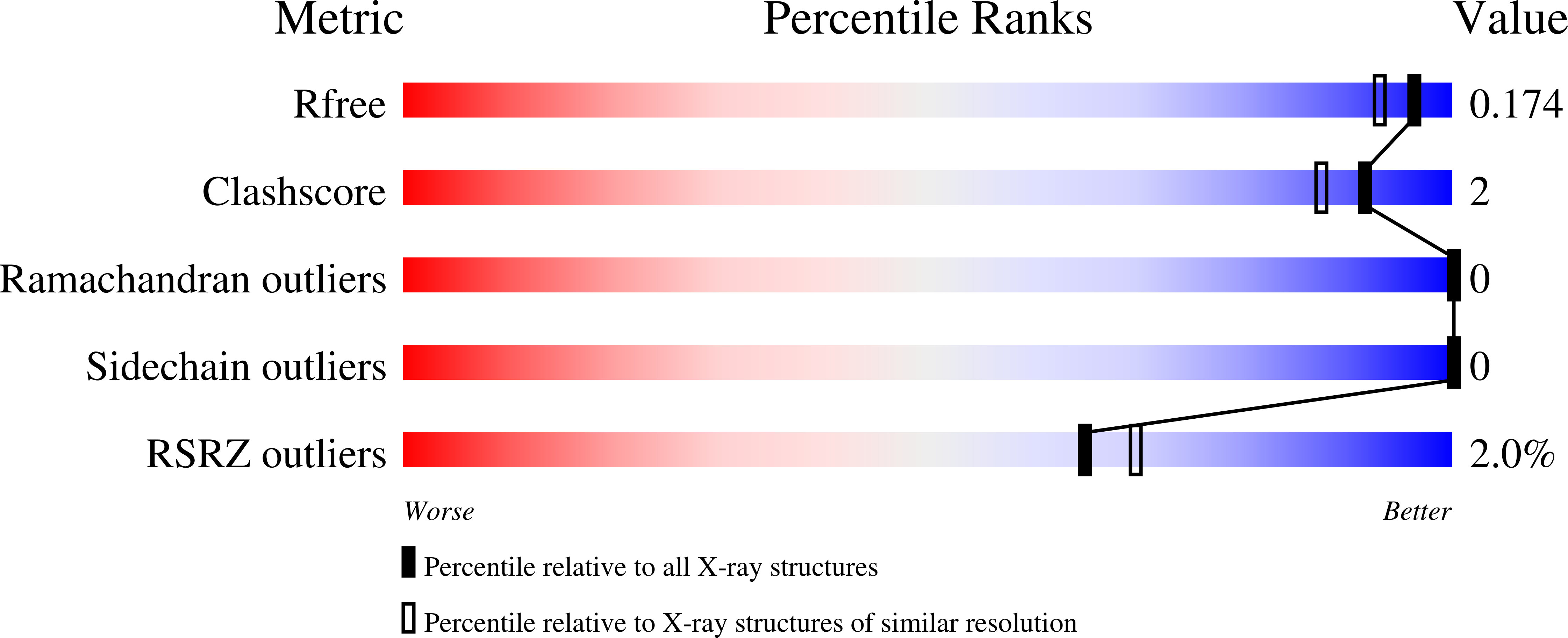
4BMH - PubMed Abstract:
The mammalian O-GlcNAc hydrolysing enzyme O-GlcNAcase (OGA) is a multi-domain protein with glycoside hydrolase activity in the N-terminus and with a C-terminal domain that has low sequence similarity to known acetyltransferases, prompting speculation, albeit controversial, that the C-terminal domain may function as a histone acetyltransferase (HAT). There are currently scarce data available regarding the structure and function of this C-terminal region. Here, a bacterial homologue of the human OGA C-terminal domain, an acetyltransferase protein (accession No. ZP_05014886) from Streptomyces sviceus (SsAT), was cloned and its crystal structure was solved to high resolution. The structure reveals a conserved protein core that has considerable structural homology to the acetyl-CoA (AcCoA) binding site of GCN5-related acetyltransferases (GNATs). Calorimetric data further confirm that SsAT is indeed able to bind AcCoA in solution with micromolar affinity. Detailed structural analysis provided insight into the binding of AcCoA. An acceptor-binding cavity was identified, indicating that the physiological substrate of SsAT may be a small molecule. Consistent with recently published work, the SsAT structure further questions a HAT function for the human OGA domain.
Organizational Affiliation:
College of Chemistry and Materials Science, Northwest University, Xi'an 710069, People's Republic of China.